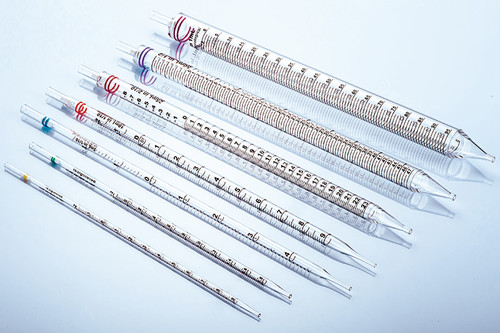
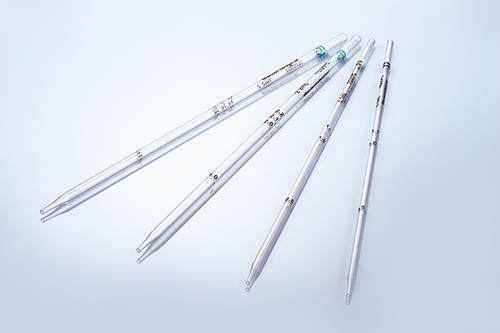
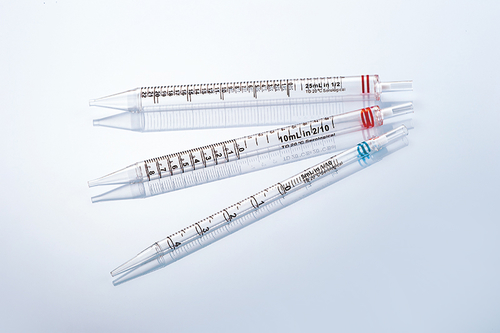
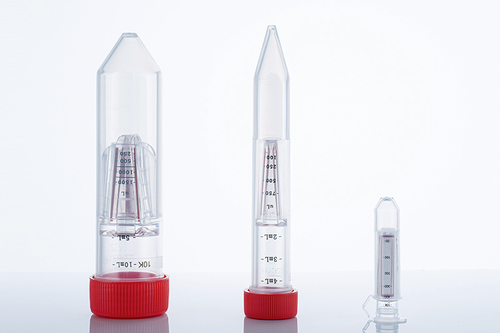
In the vast field of life science research, cell culture is the basic means of exploring the mysteries of life, and its success is directly related to the accuracy and reliability of experimental results. In this journey of exploration of the microscopic world, the importance of aseptic design and precision manufacturing process of pipettes as "precision instruments" in cell culture experiments is self-evident.
Aseptic design, as the name suggests, refers to the concept of fully considering and incorporating the prevention of microbial contamination at the beginning of product design. For serological pipettes for cell culture, aseptic design is not just a simple treatment of the product surface, but also a systematic project throughout the entire manufacturing process. It requires that every link from material selection, mold design, injection molding to final assembly must follow strict hygiene standards and aseptic operation specifications to ensure that the product reaches the medical grade aseptic state when it leaves the factory.
The realization of aseptic design is inseparable from the support of precision manufacturing technology. The manufacturing process of serological pipettes for cell culture is a collection of a series of high-precision and high-demand process flows.
The mold is the basis of injection molding, and its precision directly determines the dimensional stability and shape consistency of the pipette and its accessories. In the mold design stage, engineers will use advanced CAD/CAM technology for precise design, and use precision processing equipment to finely polish and debug the mold to ensure that the mold precision reaches the micron level. In this way, during the injection molding process, pipettes and their accessories with precise dimensions and regular shapes can be obtained.
Injection molding is a key link in the pipette manufacturing process. At this stage, it is necessary to strictly control the injection molding temperature, pressure, time and other parameters to ensure that the plastic melt can evenly and stably fill the mold cavity and form a good crystal structure. At the same time, it is also necessary to conduct strict appearance inspection and dimensional measurement of the injection molded products to eliminate defective products.
The scale of the pipette is the basis for its function realization. In order to ensure the clarity and accuracy of the scale, the manufacturer will use advanced laser engraving technology or mechanical engraving technology to form fine and uniform scale lines on the surface of the pipette. At the same time, the scale will be calibrated and verified by precision measuring equipment to ensure that the pipette can accurately and stably read the liquid volume during use.
In addition to the precise manufacturing process, a strict quality control system is also an important guarantee to ensure the aseptic design and quality of the pipette. In the manufacturing process of the pipette, every link must undergo strict quality inspection to ensure that all indicators of the product meet the design requirements and national standards.
To ensure the sealing and sterility of the gun tip and the pipette, the manufacturer will conduct a series of special tests. Among them, the airtightness test is used to evaluate the sealing performance between the gun tip and the pipette to prevent liquid leakage during the absorption and discharge process. The microbial limit test is used to detect whether there is microbial contamination on the surface and inside of the product to ensure the sterility of the product. In addition, pressure resistance tests and corrosion resistance tests will be carried out to comprehensively evaluate the performance and quality of the pipette.
In order to ensure that each pipette can be traced back to its production source and every detail in the manufacturing process, the manufacturer will establish a complete quality traceability system. By assigning a unique identification code or serial number to each pipette and associating it with various records in the production process, the full chain quality traceability of the product from raw material procurement, production and processing to finished product delivery can be achieved. In this way, when quality problems occur, the source of the problem can be quickly located and effective corrective measures can be taken.
Through the combined effect of the above-mentioned precision manufacturing process and strict quality control system, the serological pipettes for cell culture and their accessories have reached the medical grade sterility state when they leave the factory. This not only provides a safe and reliable experimental environment for cell culture experiments, but also greatly reduces the risk of experimental failure caused by microbial contamination during the experiment. At the same time, the precise pipetting function of the pipette also provides great convenience and accuracy for liquid handling in cell culture experiments.
The aseptic design and precision manufacturing process of serological pipettes for cell culture are important foundations for ensuring the success of experiments and the reliability of results. In future life science research, with the continuous advancement and innovation of technology, we have reason to believe that the aseptic design and manufacturing process of pipettes will be more perfect and optimized, providing more efficient, accurate and reliable support for cell culture experiments.

 English
English 中文简体
中文简体